Research & Development

Our Lead Candidate
The innate and the adaptive arms of the human immune system play a critical role in host defense, orchestrating immune recognition and elimination of pathogens or malignant cells. In oncology settings, tumours have evolved mechanisms that facilitate immune escape and promote tumour progression and metastasis. First generation immunotherapy agents targeting CTLA-4 and PD-1/PD-L1 checkpoint pathways were designed to re-engage or enhance the adaptive immune response and have demonstrated significant impact in clinical trials. However, not all patients display response to checkpoint blockade and significant unmet medical need remains.
AXA-042 is a novel synthetic systemic TLR2/6 agonist designed to re-engage the innate immune response pathways to help overcome tumour immune escape.
Toll-Like Receptors (TLRs) are a family of proteins that recognize pathogen associated patterns (PAMPs) and primarily function to activate the innate immune response. TLR2 is a membrane bound receptor, predominantly expressed on the cell surface of myeloid cells, including dendritic cells, macrophages and neutrophils, as well as NK cells and activated T cells. Depending on ligand context, TLR2 heterodimerizes with either TLR1 or TLR6 to promote expression of key pro-inflammatory cytokines and chemokines.
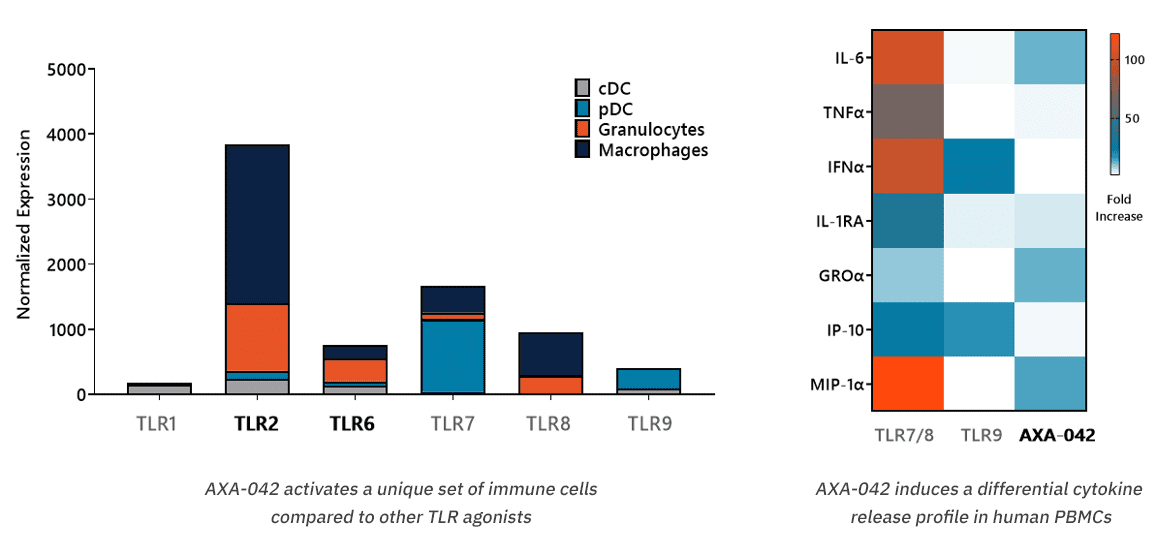
Our research has led to the identification of a novel chemical entity, AXA-042 which selectively stimulates TLR2/6 heterodimers and displays a unique PBMC cytokine release profile compared to other TLR agonists. AXA-042 does not potentiate significant IFNα or TNFα release, typically associated with the onset of the Cytokine Release Syndrome (CRS).
Differential AXA-042 Target Expression and Cytokine Release Profile
AXA-042 acts early in the cancer immunity cycle, activating antigen presenting cells and promoting Th1 cytokine release to recruit T-cells to the tumour microenvironment. Furthermore, AXA-042 alters the activation status of tumour associated macrophages and neutrophils to further potentiate tumour cell death and antigen release. AXA-042 has been extensively profiled across a number of murine syngeneic tumour models, demonstrating activation of the innate immune response and reduction of tumour burden as monotherapy and in combination with established checkpoint inhibitors. AXA-042 whole blood and tumour response signature has been identified and shown to be conserved across different preclinical models and species.
AXA-042 Alters the Tumour Microenvironment and Enhances Checkpoint Inhibitor Anti-Tumour Response
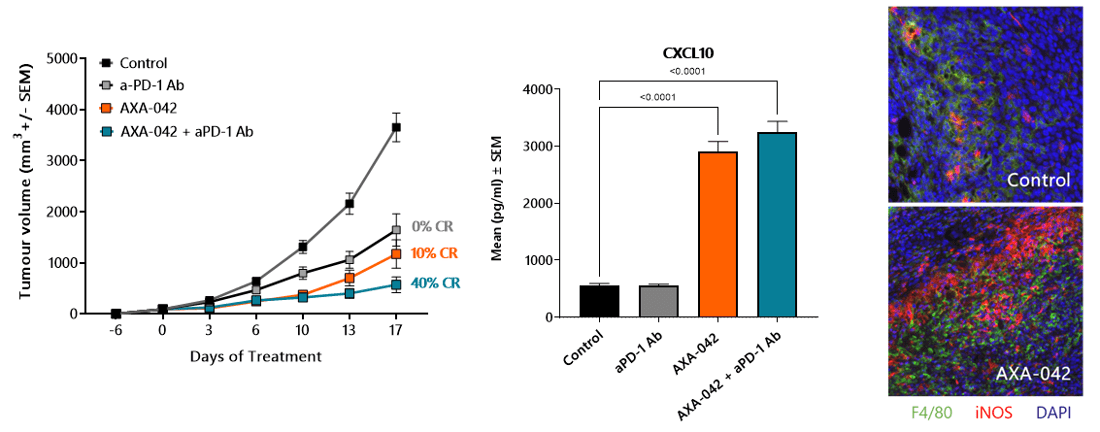
- Once-a-week AXA-042 treatment inhibits CT26 tumour growth in vivo
- AXA-042 potentiates anti-PD-1 Ab treatment response, leading to 40% complete regressions
- Efficacy is accompanied by a re-polarization of the tumour microenvironment and increased IFNγ signature
AXA-042 has completed GLP toxicology studies.